Immune Tolerance Induction for Hemophilia A Patients
Immune Tolerance Induction for Hemophilia A Patients with Inhibitors
Around one in three previously untreated hemophilia A patients develops neutralizing antibodies (inhibitors) against factor VIII (FVIII), rendering therapy ineffective.1-3 This is the most serious and challenging complication of FVIII therapy in hemophilia A.2 The resulting resistance to therapeutic FVIII may have major adverse consequences for disease management and outcomes.4
Immune Tolerance Induction: A Proven Tool for Inhibitor Management
Immune tolerance induction (ITI) is the only proven approach for eradicating inhibitors and increasing patient tolerance to FVIII. The treatment involves prolonged, daily exposure to high-dose FVIII.5 ITI has been shown to eradicate inhibitors in 60%-90% of patients with hemophilia A.5
The success rate of ITI may vary depending on patient variables and factors relating to the pattern of treatment for the induction of immune tolerance.6
Both plasma-derived (pd) or recombinant (r) FVIII ITI are recommended as the primary treatment option to eradicate inhibitors in both European and US guidelines.4,5 However, some recent studies on both children and adults suggest that using pd FVIII with a high presence of von Willebrand factor (VWF) may exert an immunoprotective effect on the FVIII, which may consequently have a positive impact on the success of the ITI.6,7,8
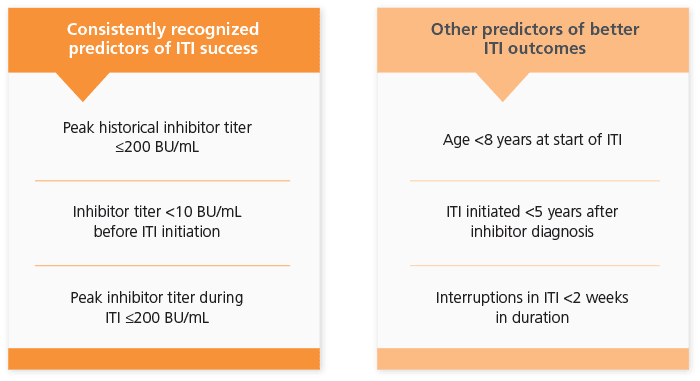
Favorable Risk Factors for ITI Success5
Typical ITI Dosing5
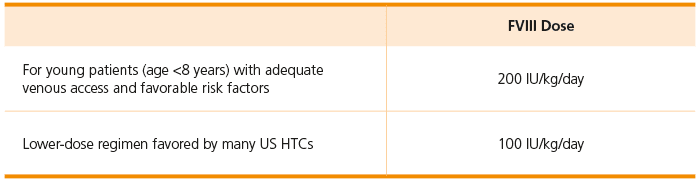
- Lower-dose regimens are supported by cohort and registry data; no randomized clinical trials. HTCs may use internal protocols and guidelines for higher-dose regimens
- MASAC (Medical and Scientific Advisory Council) recommendations on concomitant use of Hemlibra® (emicizumab) during ITI suggest no more than 50 IU/kg per dose be administered unless observation occurs within a clinical trial9
Using wilate for ITI Therapy
wilate is a high-purity human pdFVIII/VWF concentrate containing the native FVIII/VWF complex in a physiological 1:1 ratio.10 A recent study reports favorable results using wilate for ITI in children with severe hemophilia A with inhibitors.4
Study design:4
- Conducted in 11 severe hemophilia A patients with inhibitors
- 2 patients were treated with wilate for rescue ITI (previous ITI treatment was unsuccessful)
- All but 1 patient had at least one poor prognosis factor for ITI outcome
- All but 3 patients were less than 6 years of age at onset of ITI with wilate
- Titers were measured prior to and during ITI (Nijmegen-Bethesda)
- Patients were treated with wilate ITI doses of 50-60 IU/kg, 3x/week, and up to 200 IU/kg/day
Outcomes:
- Complete or partial success in 9 of 11 (82%) patients— including two patients who received rescue ITI 4
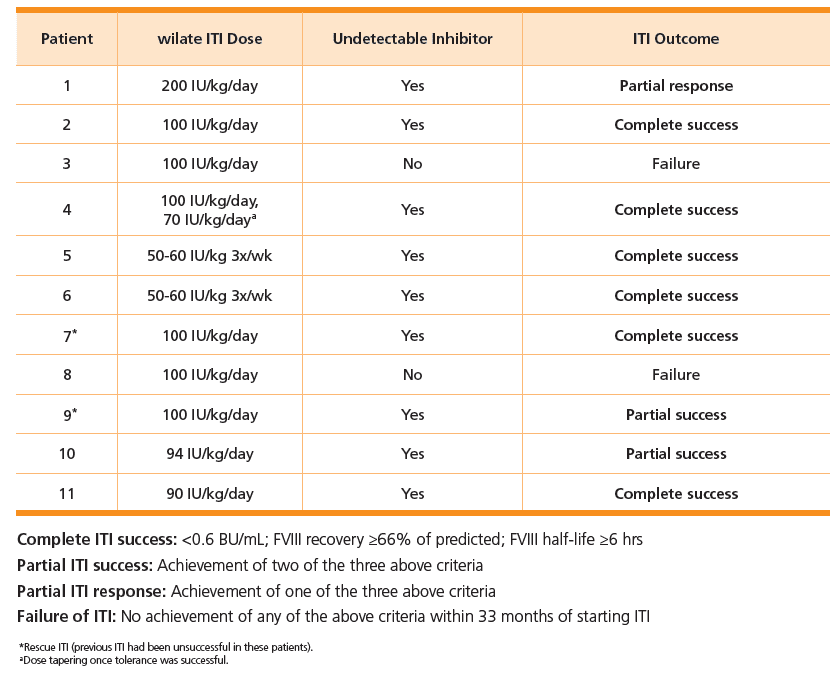
- Low bleeding rates while on wilate ITI4
- Median ABR (annual bleed rate) decreased by 35% during ITI with wilate compared with the time with inhibitors before ITI.
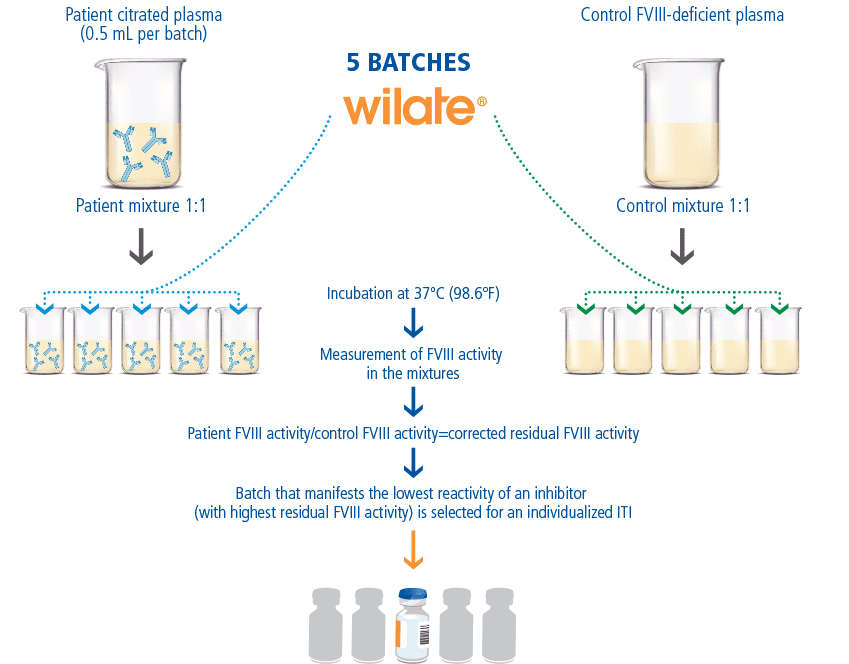
Wilate Batch Selection to Optimize ITI Therapy
When treating patients with inhibitors, different interactions and effects can occur between the inhibitors and a given batch of factor concentrate. For this reason, Octapharma offers wilate prescribers the option of batch selection.11 Batch (or lot) selection involves measuring residual FVIII activity when patient plasma is mixed with different lots of wilate in vitro.12 By performing an inhibitor assay (New Oxford Assay) with several batches of wilate, patients can receive the wilate batch (or lot) with the lowest Oxford Unit (OU) and highest residual FVIII, as the optimal choice for ITI.11
Wilate Is the Only VWF/VIII Product Offering Batch Selection
References
- Vézina, C., Carcao, M., Infante‐Rivard, C., Lillicrap, D., Stain, A.M., Paradis, E., Teitel, J., Rivard, G.E. and (2014), Incidence and risk factors for inhibitor development in previously untreated severe haemophilia A patients born between 2005 and 2010. Haemophilia, 20: 771-776. doi:10.1111/hae.12479
- Xi, M, Makris, M, Marcucci, M, Santagostino, E, Mannucci, PM, Iorio, A. Inhibitor development in previously treated hemophilia A patients: a systematic review, meta‐analysis and meta‐regression. J Thromb Haemost 2013; 11: 1655– 62. DOI:10.1111/jth.12335.
- Blanchette, VS, Key, NS, Ljung, LR, Manco‐Johnson, MJ, van Den Berg, HM, Srivastava, A, for the Subcommittee on Factor VIII, Factor IX and Rare Coagulation Disorders. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost 2014; 12: 1935– 9.
- Belletrutti M, et al. (2020). Immune tolerance induction using wilate, a human von Willebrand factor/factor VIII concentrate, in patients with severe haemophilia A and inhibitors: A retrospective study of 11 children in two Canadian haemophilia treatment centres. Manuscript in preparation
- Valentino, L.A., Kempton, C.L., Kruse‐Jarres, R., Mathew, P., Meeks, S.L., Reiss, U.M. and (2015), US Guidelines for immune tolerance induction in patients with haemophilia a and inhibitors. Haemophilia, 21: 559-567. doi:10.1111/hae.12730
- Oldenburg, J., Jiménez‐Yuste, V., Peiró‐Jordán, R., Aledort, L.M. and Santagostino, E. (2014), Primary and rescue immune tolerance induction in children and adults: a multicentre international study with a VWF ‐containing plasma‐derived FVIII concentrate. Haemophilia, 20: 83-91. doi:10.1111/hae.12263
- Gringeri A, Musso R, Mazzucconi MG et al. Immune tolerance induction with a high purity von Willebrand factor/VIII complex concentrate in haemophilia A patients with inhibitors at high risk of a poor response. Haemophilia 2007; 13: 373–9.
- Kurth, M., Puetz, J., Kouides, P., Sanders, J., Sexauer, C., Bernstein, J., Gruppo, R., Manco-Johnson, M., Neufeld, E.J., Rodriguez, N., Wicklund, B., Quon, D. and Aledort, L. (2011), The use of a single von Willebrand factor‐containing, plasma‐derived FVIII product in hemophilia A immune tolerance induction: the US experience. Journal of Thrombosis and Haemostasis, 9: 2229-2234. doi:10.1111/j.1538-7836.2011.04493.x
- Recommendation on the use and management of emicizumab-kxwh (Hemlibra) for hemophilia A with and without inhibitors. Medical and Scientific Advisory Council (MASAC) of the National Hemophilia Foundation (NHF) on November 21, 2018; adopted by the NHF Board of Directors on December 6, 2018. MASAC Document 255.
- wilate Full Prescribing Information. Paramus, NJ: Octapharma; rev December 2023.
- Data on file, Octapharma. 2015-2017.
- Thornburg, C.D. and Ducore, J. (2019), A novel approach to immune tolerance induction in haemophilia A with factor VIII inhibitor. Haemophilia, 25: e48-e50. doi:10.1111/hae.13646
